Vaginosis and consequences of child and maternal care
Introduction
The lower female reproductive tract, specifically the vagina and ectocervix, is considered a formidable chemical and physical barrier to exogenous invading organisms, in part due to the structure of the stratified vaginal epithelium and the presence of cervicovaginal fluid (CVF). Eubiotic viscoelastic CVF acts as an effective lubricant, facilitates the trapping of exogenous organisms and, acts as an acidified medium in which there is an arsenal of antimicrobial molecules (antibodies, defensins etc.). Importantly, this mucosal layer (mucus and layers of dead epithelial cells) also enables the adhesion of mutualistic vaginal microbiota1).
The physical barrier of its lining is composed of stratified squamous epithelium that, unlike the upper reproductive tract, relies primarily on the presence of multiple layers to provide a protective barrier against the entry of organisms. However the outermost layers are terminally differentiated (cornification) and lack many of the intracellular organelles including nuclei. They form a network of loosely connected glycogen-filled cells while the basal layer is metabolically active and undergoes active proliferation and is sealed by tight junctions. Consequently, the superficial layers of the lower genital tract are quite ‘leaky' and permeable to water and soluble proteins, allowing penetration by endogenous and pathogenic microbes and molecular and cellular mediators of innate and adaptive immune defense.
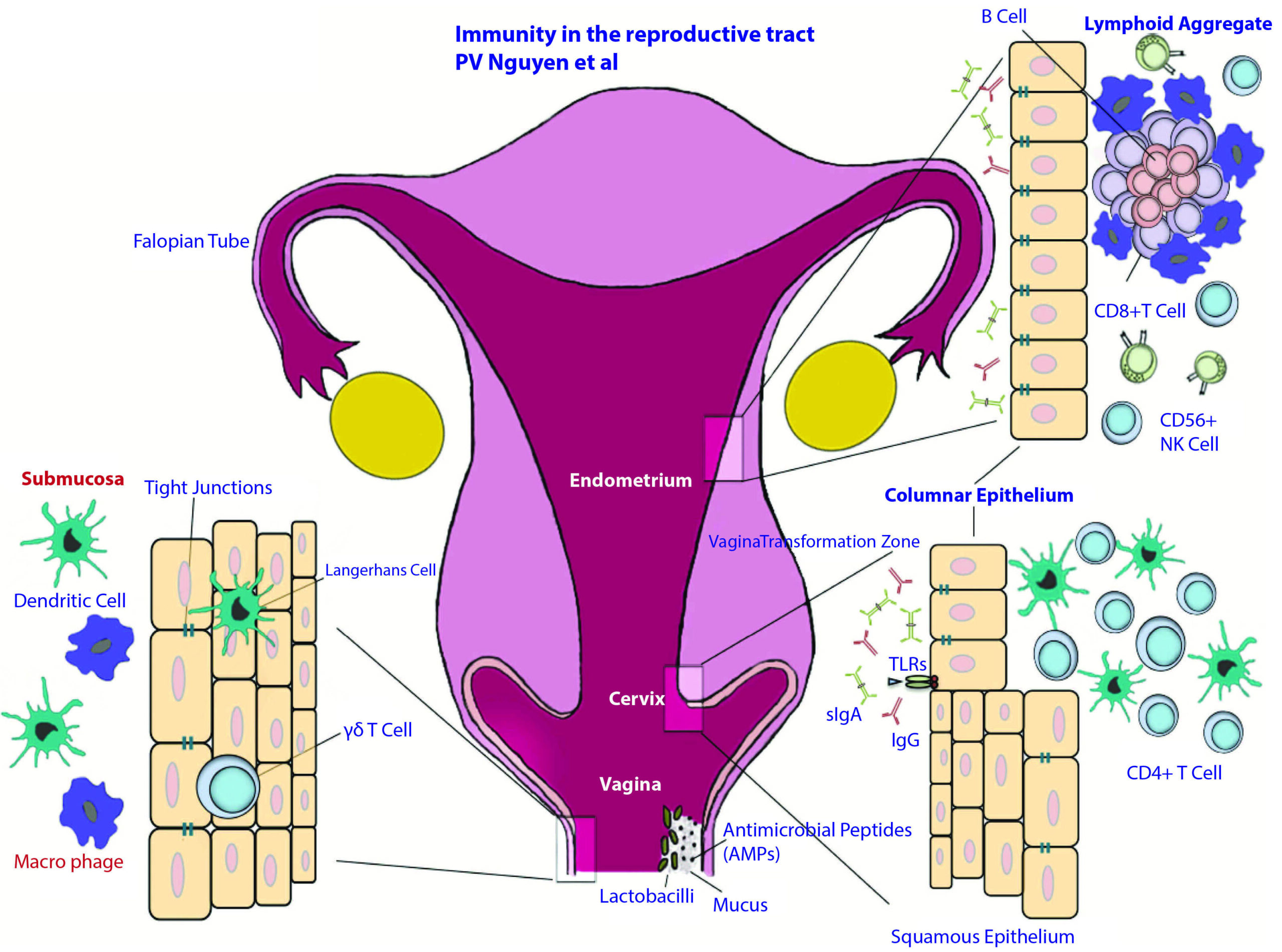
Figure 1
Anatomical and immunological components of the female reproductive tract.
The female reproductive tract consists of an upper (fallopian tubes, uterus and endocervix) and a lower (extocervix and vagina) tract. The vaginal epithelium has many innate immune-mediated protection mechanisms, such as tight junctions, AMPs and mucus, to neutralize, trap, and prevent the entry of potential pathogens. The vaginal lumen is colonized by commensal bacteria, mainly Lactobacilli spp., which help maintain a low pH environment and produce reactive oxygen species. Furthermore, innate immune cells, such as cd T cells, DCs, and macrophages, are present beneath and between vaginal epithelial cells layer to survey the local environment for danger. The abrupt transition from keratinized squamous epithelial cells of the ectocervix to single columnar epithelial cells of the endocervix represents the transformation zone; this site has an abundance of HIV target cells (DCs and CD41 T cells) and has been proposed to be one of the major sites of infection. Although traditional mucosal lymphoid structures are not found in the female reproductive tract, lymphoid aggregates in the endometrial tissue that are composed of B cells in the inner core and surrounded by CD81CD42 T cells and an outer layer of macrophages have been described. Scattered CD561 NK cells and CD41 T cells can be found between lymphoid aggregates. The immune cells and functions of the female reproductive tract are regulated by sex hormones that orchestrate cyclical changes with the menstrual cycle. AMP, anti-microbial peptide; DC, dendritic cell; NK, natural killer2).
The suprabasal epithelial layers of the ecto-cervix have a rapid turnover so that desquamation seems to be a feature of this zone. Although unknown, the rate of desquamation may be affected by intercourse, use of vaginal products, and hormonal status. Exfoliation is an effective way to eliminate pathogens that have attached to the vaginal surface or that bind to sloughed “decoy” cells. A 3H-thymidine uptake study showed that the minimum transit time of labelled keratinocytes from the basal layer of the human vaginal epithelium to the uppermost layer was 96 hours; since this epithelium is approximately 28 cell layers thick, this study provides evidence that one cell layer is lost from the vaginal epithelium approximately every 4 hours3).
This innate mechanism apt in shedding pathogens that excludes them from sojourning in the epithelial surfaces demands a special microenvironment in order to compensate for the consequent leakiness of the epithelial barrier and preserve protection from pathogens.
The vaginal microenvironment is a very singular subject matter that must be understood in order to envision protection against sexually transmitted infections, prevention of adverse reproductive outcomes and eventually for insight in devising valuable appropriate measures that can remediate these unfavorable states of disease. Moreover as it has become increasingly clear over time there is no “bacterial signature” (Vide Dysbiosis) when in dealing with them antibacterial measures with antibiotics are adopted nor a clear “metabolic signature” has been identified for establishing reliable diagnostic markers, so that this herein new approach emerges as a “new window of opportunity” in order to harness efficacious corrective measures. Thus the aforesaid subject will be dealt with in the following sections.
Vaginal microenvironment
Hypoxia
Previous studies have ignored the high carbon dioxide (CO2) and low oxygen (O2) levels that prevail in the vaginal environment. The cervicovaginal partial pressure of CO2 is equivalent to systemic levels (38 mm Hg or 5%)[46]; this CO2 is rapidly lost when the vaginal lumen or a CVF sample is exposed to air (partial pressure of CO2 in air ~ 0.30 mm Hg or 0.04%). Furthermore, the cervicovaginal partial pressure of O2 is in the hypoxic range of 4-14 mm Hg (2%), with only transient increases due to insertion of a tampon or diaphragm, sexual arousal and, presumably, vaginal intercourse4).
Glycogen, pH and Lactobacillus
A relationship between vaginal epithelial glycogen and Lactobacillus colonization in the female lower genital tract has been recognized for many decades. Thus, Cruickshank5) and others6)7) observed that at puberty, glycogen becomes expressed in the vaginal epithelium and at the same time colonization with Lactobacillus becomes apparent. Those studies showed that both glycogen expression and colonization by Lactobacillus persist through life until at menopause, when both Lactobacillus colonization and glycogen expression decline. Lately it has become evident that even for low levels of glycogen in premenopausal and postmenopausal women the association is strictly conserved being relatively lactobacilli more abundant in premenopausal than in postmenopausal women and this presence intimately related to the amount of free glycogen present8). This association between colonization with Lactobacillus and deposition of intraepithelial glycogen has led to the hypothesis that glycogen serves as an important energy source for lactobacilli and their ability to colonize and produce lactic acid in the female lower genital tract 9)10).
An eco-niche metabolically dependent on the formation of lactic acid becomes relevant. But lactobacilli alone will not achieve the low pH that characterizes this microenvironment. Despite the evidence that glycogen promotes colonization by lactobacilli, most isolates of Lactobacillus do not grow in vitro in media containing glycogen as the carbohydrate source. Thus, Stewart-Tull 11) reported in 1964 that a number of isolates of Lactobacillus did not ferment glycogen. Later in that decade, Wylie and Henderson 12) showed that 39 of 42 isolates of Lactobacillus did not ferment glycogen, even when the glycogen was isolated from the genital tract of women. More recently, Martin et al 13) showed 45 strains of vaginal Lactobacillus could not use glycogen. The lack of Lactobacillus growth in vitro in the presence of glycogen leaves open the question of how glycogen could be utilized in vivo by Lactobacillus.
A study by Spear et al. (2004) supports a model where α-amylase present in the genital tract breaks down glycogen into smaller polymers, such as maltose and maltotriose, that are then utilized by Lactobacillus to aid its colonization. Isolates of Lactobacillus that cannot use glycogen can grow in glycogen breakdown products. An activity that breaks down glycogen to maltose and maltotriose was demonstrated in the study to be present in the lower-genital-tract fluid of women: glycogen breakdown activity in genital fluid correlates with genital levels of α-amylase; incubation of genital fluids with glycogen generated products such as maltose, maltotriose, and maltotetraose. The study therefore supports presence of α-amylase in the genital tract which breaks down glycogen into smaller polymers, such as maltose and maltotriose, which are then utilized by Lactobacillus to aid its colonization14).
Although free glycogen levels varies greatly between women and even in the same woman, samples in a study with the highest free glycogen had a corresponding median genital pH that was significantly lower (pH 4.4) than those with low glycogen (pH 5.8; p<0.001). The fraction of the microbiota consisting of Lactobacillus was highest in samples with high glycogen versus those with low glycogen (median = 0.97 vs. 0.05, p<0.001)15).
Control Redox
Although redox chemistry life itself on earth for over 3.5 billion years it is wisely underestimated or misunderstood. In the whole literature there are only two references related to what redox microenvironment can be found in the vagina. Holmes et al. (1985) while investigating the altered metabolic profile measured the redox potential (Eh) at the vaginal epithelium. It was measured in healthy asymptomatic women and women with bacterial Vaginosis BV (Vide Dysbiosis). Women with BV were reported to have a more reduced vaginal environment compared to healthy asymptomatic women. Typically, redox potential varies by ± 60 mV per pH unit but the difference between the two groups was 262 mV for 1 pH unit difference, indicating that pH alone could not account for the altered redox potential. In healthy women it ranged +322 mV to +137 mV. In women with BV +71 mV to as low as −257 mV. The difference in redox potential was attributable to microbial metabolism rather than a host determinant because antibiotic treatment successfully increased the redox potential, similar to that of healthy asymptomatic women16).
Notably, a more reduced vaginal environment is conducive to the growth of anaerobes, which increase in number and diversity during BV. It was hypothesized by (Holmes et al., 1985) that vaginal microbiota would influence the redox potential due to altered concentrations of organics acids that form redox pairs (e.g., lactate/pyruvate and succinate/fumarate). However the redox potential changes are due to the respiratory activity of anaerobic bacteria which in a favorable environment use oxidized species as electron aceptores in the respiratory chain perturbing the redox microenvironment. The oxidative stress and erosion in the epithelial barrier are quite evident as demonstrable by metabolomic studies (Vide Metabolomics), providing abundant electron acceptors species and most notably host free iron Fe+++ or iron extracted from metal containing compounds17). The hypoxic environment in the vagina indubitably favors the aforementioned anaerobic growth.
Oxidative stress co-exists with a perturbed reduced environment. Fact highly demonstrative of oxidative stress being more the disruption of physiological redox signaling events and why it cannot mostly be simply remedied with antioxidants; “antioxidant paradox”18). Ratios of reduced glutathione (GSH) to oxidized glutathione (GSSG) speak of the redox environment. Decrease in the GSH/ GSSG ratio is suggestive of oxidative stress due to its link to protein structure through kinetically controlled redox switches in the proteome and eventual protein irreversible damage. In the second study found on cervicovaginal redox state, the GSH/GSSG ratio in women without BV was found to be 3.29, and that in women with BV was 0.23. Similar ratios were noted in the validation study of the latter. All BV-associated bacteria (detected by qPCR) and Lactobacillus iners were negatively correlated with the presence of GSH. Lactobacillus crispatus and Lactobacillus jensenii were positively correlated with the presence of GSH. In addition, GSH was negatively correlated with the presence of clue cells. Another metabolite indicative of oxidative stress is ascorbate (vitamin C), a major antioxidant whose levels were significantly lower in BV19).
Vaginal Microenvironment Definition
The vagina is an hypoxic mucosal cavity protected by the cervicovaginal fluid and immunological secretions of stratified squamous epithelium in an acid medium hosting a Lactobacillus dominated microbiota and a Eh ranging between +322 mV to +137 mV.
Dysbiosis
Perturbation of the above environment favors a polymicrobial imbalance of the vaginal microbiota, or dysbiosis, where predominance of harmful bacteria create an altered environment susceptible of harboring bacterial vaginosis BV. This facilitates contagion of sexually transmitted diseases and the spread of BV-associated bacteria ascending infection into the upper female genital tract, urinary tract and pelvis.
Gram-stain-based methods described by Nugent 20) and Spiegel 21) have been used to delineate BV from other vaginal disorders or infections (vaginal candidiasis/trichomoniasis, gonorrhoeae, chlamydia). These methods apply a numbered ranking system from 1–10, where an increase reflects reductions in the relative proportions of Lactobacillus morphotypes and increases in Gardnerella and other Gram-variable rod shaped morphotypes. The initial method of Spiegel was evaluated by Nugent to be only slightly better than chance in predicting BV, while Nugent and colleagues reported an almost 20% false positive rate in describing an improved approach. Recent studies suggest just 16%–37% of women with a Nugent score indicative of BV actually show symptoms of the disease 22). A likely confounding factor of these approaches is that reductions in lactobacilli are also occasionally seen in asymptomatic individuals 23), 24) and G. vaginalis is commonly found in the vaginal tract, regardless of disease symptoms 25)26). A more definitive diagnosis is based on the Amsel criteria 27), which diagnoses BV based on the presence of three of the four symptoms (discharge, odor, pH>4.5 or clue cells). Because of the limitations associated with the Nugent method, Amsel criteria are more commonly employed in a clinical setting and CDC guidelines preclude treatment without reported or observed symptomology. Despite this, Nugent score continues to be used in research as a diagnostic tool and this may have confounded our current understanding of BV.
The diversity of the vaginal microbiome among each individual complicates the ability to determine a “bacterial signature” of BV from that of the temporal changes in the vaginal microbiome due to reproductive cyclicity, personal hygiene or other numerous variables. This temporal fluctuation of the vaginal microbiome makes it difficult to identify bacterial species that are causative for BV or the invasion of opportunistic bacterial species that colonize and populate the vaginal microbiome during environmental changes of the vaginal cavity. The difficulty in identification of a causative pathogen for BV also increases the difficulty of developing effective treatments for BV.
Treatment protocols for BV include antibiotic therapy either with oral tablets or vaginal creams 28). Additionally, the utilization of combo or progestin only oral contraception drugs (OCPs) has shown a protective effect to development of BV through hormonal stabilization of microbial communities in the vaginal canal 29). However, reoccurrence of disease within 1 year following the cessation of treatment is high 30)31)32)
BV poses current medical challenges associated with diagnosing and treating the disease. Diagnosis is particular difficult in patients who are asymptomatic 33)34). Treatment of disease (anti-microbial vs. probiotics) is quite shortsighted and specially during pregnancy: reviewed in 35)36). It fully challenges classical precepts of causality of disease since it is another example of many that do not fulfill Koch's postulates of infectious disease causality 37). Thus, even centuries since its discovery, BV still remains a nuisance for scientists and clinicians worldwide since if not controlled it becomes a risk factor for the development of other abnormal health conditions, which can be either confined or will extend far away in the female genital tract and yonder38)39).
Metabolomics
Yeoman et al. using a multi-omics approach found in lavage samples from 36 BV symptomatic women that 16S rRNA gene-based community composition profiles reflected Nugent scores, but not Amsel criteria. In contrast, metabolomic profiles were markedly more concordant with Amsel criteria. Two discrete subgroups within each metabotype were characterized and referred to as SBVI and SBVII40). Srinivasan et al. identified in women with BV, at least two subtypes based on the metabolic profiles, reflecting different concentrations rather than the presence/absence of particular metabolites41).
Many of the metabolites significantly affected in samples from women with a high Nugent score or displaying symptoms of BV types were fatty acids, including both SCFA and those with longer chain moieties. These and other metabolites, found to be diminished in SBVI and SBVII (such as ethanolamine, glycerol, serine, phosphate), are related to glycerophospholipid metabolism and are highly suggestive of either reduced glycerophosphodiester phosphodiesterase-activity 42) or rapid utilization of these and other host cell membrane degradation bi-products (such as cholesterol; 2-aminoethylphosphate which were also significantly decreased) in the symptomatic BV state. Correlates with the latter hypothesis, the increases in the biomembrane lipid anchor, tetradecanoic acid, an essential structural component of host cell membranes and their derivatives, was observed in the vaginal metabolomes of SBVI patients. Significant differences in amino acids characteristic of integral membrane proteins were also noticed. Amino acids with aliphatic side-chains, predominant in the transmembrane regions spanning the middle of the phospholipid bilayer (leucine, isoleucine, alanine and valine) were all depleted in metabolome samples from women with SBVI and/or those with a high Nugent score. While the amino acids tyrosine and tryptophan, which are typically located at the polar/non-polar interface of transmembrane protein regions were depleted in samples from women with high Nugent scores. The finding that these observations overlap with Nugent scores implies an important role for G. vaginalis and other Nugent-defined morphotypes in the reduction of cell wall integrity. In accord with this, the genomes of two G. vaginalis strains isolated from women with symptomatic BV harbor mucinolytic enzymes 43).
Consistent with the above is the increased sialoglycan degradation present in human vaginal specimens. It has been demonstrated that Gardenella vaginalis engaged in sialoglycan foraging in vitro, in the presence of human vaginal mucus, and in vivo, in a murine vaginal model, in each case leading to depletion of sialic acids. Comparison of sialic acid levels in human vaginal specimens also demonstrated significant depletion of mucus sialic acids in women with BV compared to women with lactobacilli-dominated microbiota. G. vaginalis utilizes sialidase to support the degradation, foraging, and depletion of protective host mucus barriers of the vaginal mucosa44).
A point-of-care diagnostic test for BV that is currently available and relies on the measurement of sialidase activity in vaginal fluids45): It is useful since it is simple and quick. However it doesn't rule out other infections. On the same token measuring the Eh of cervicovaginal fluid would provide analogous information to complement Amsel's diagnostic criteria.
The most significant finding from metabolomics studies is the evidence pointing to BV debilitation of the epithelial barrier and tight junctions favoring sexually transmitted diseases or the endogenous ascending anaerobic infection towards the upper female genital tract, pelvis and the urinary tract. Interventions for BV recommended in this essay will emphasize reconstituting the epithelial barrier and its associated mucosal immunity defenses (generally underestimated) in a robustly balanced healthy microenvironment; entirely competent and sufficient in deterring the adverse events mentioned above. Therefore the identification of pathogens and deriving classifications46) become of taxonomic relevance only and, on the contrary, rather than trying to eliminate individualized bacteria (purported pathogens) the mutualist microbiota aspects might be more meaningful in deriving yet-to-be-discovered measures that may be complementary in fostering symbiosis.
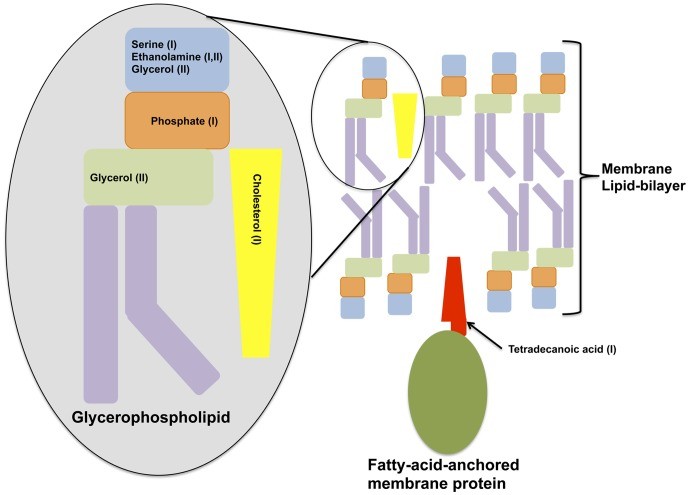
Figure n° 2
Cell Membrane Degradation
Shown is a diagram depicting classical components of a cell membrane that is depleted in either of the metabolome profiles of the two symptomatic BV types.
Symptoms association with metabolites
The usefulness of metabolic studies has resided more on setting firm associations between metabolites and symptoms of BV (Amsel criteria)rather than classifying subgroups of BV. In the figure below such associations are presented as ascertained by Srinivasan et al. 47) using penalized linear regression models.
(Yeoman et al. 2013)48) found the symptoms ‘itching’ and ‘vaginal pain’ to be linked (Spearman’s R=0.64) to each other, as well as to diethylene glycol (Spearman’s R.0.6) and several distinct metabolites.
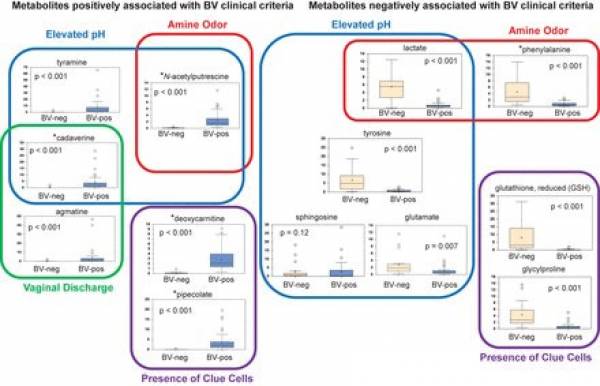
Figure n° 3
Association of metabolites with Amsel clinical criteria. Shown is a model depicting metabolites associated with individual clinical criteria used in BV diagnosis . Stars denote metabolites that were positively or negatively associated with BV status. BV status is indicated on the x axis of box plots. The y axis of box plots represents scaled concentrations of metabolites. The lines in box plots represent the mean, and whiskers denote 95% confidence intervals.
Microenvironment control proposal
The microenvironment definition above sets the parameters in correcting the dysbiosis present in BV.
The challenge in the vaginal microenvironment is the acidic pH which is to be kept around 4 or preferably more towards 3.5 49). Paradoxically elevating pH will not help correct the overgrowth of anaerobes. As seen by (Holmes et al. 1983)50) one pH unit variance changes disproportionately Eh towards reducing conditions in the vagina (Vide Control Redox). Therefore the redox buffering aspect is determinant and restitution of oxidative redox potential is imperative.
However Gynactil's pH is 4 and the acid/base buffering capacity will maintain stable such environment due to the alkali absorbing properties of the system. The redox couple has a +183 mV potential.
This formulation has proven to be highly effective in controlling BV. It restitutes libido depressed in patients affected by BV, Also it has shown to regularize menstruation eliminating discomfort associated with it and dysmenorrhea.
Due to stability regarding vulvar/vaginal status in women using Gynactil regularly and due to women carrying a disproportionately higher burden of sexually transmitted diseases it should be interesting in conducting studies in the prevention of cervical cancer51) and HIV contagion52) with this new microenvironment control strategy.
BV has been associated with increased risk of preterm delivery, first-trimester miscarriage in women undergoing in vitro fertilization, preterm premature rupture of membranes, chorioamnionitis, amniotic fluid infections, postpartum and postabortal endomyometritis as well as postabortal pelvic inflammatory disease (PID). All problems that can be solved with the microenvironment control methodReferences
1) Aldunate, M., Srbinovski, D., Hearps, A. C., Latham, C. F., Ramsland, P. A., Gugasyan, R., … Tachedjian, G. (2015). Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Frontiers in Physiology, 6, 164. http://doi.org/10.3389/fphys.2015.00164
2) Nguyen, P. V., Kafka, J. K., Ferreira, V. H., Roth, K., & Kaushic, C. (2014). Innate and adaptive immune responses in male and female reproductive tracts in homeostasis and following HIV infection. Cellular and Molecular Immunology, 11(5), 410–427. http://doi.org/10.1038/cmi.2014.41
3) Averette, Hervy E. et al. (1970) Autoradiographic analysis of cell proliferation kinetics in human genital tissues American Journal of Obstetrics & Gynecology , Volume 108 , Issue 1 , 8 - 17 http://www.researchgate.net/publication/51267609_Autoradiographic_analysis_of_cell_proliferation_kinetics_in_human_genital_tissues._I._Normal_cervix_and_vagina._Am_J_Obstet_Gynecol
4), 49) O’Hanlon, D. E., Moench, T. R., & Cone, R. A. (2013). Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE, 8(11), e80074. http://doi.org/10.1371/journal.pone.0080074
5), 10) Cruickshank, R. (1934), The conversion of the glycogen of the vagina into lactic acid. J. Pathol., 39: 213–219. doi: 10.1002/path.1700390118 http://onlinelibrary.wiley.com/doi/10.1002/path.1700390118/abstract
6) Harald et al. (2003) Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis Leitich, American Journal of Obstetrics & Gynecology , Volume 189 , Issue 1 , 139 - 147 http://www.ncbi.nlm.nih.gov/pubmed/12861153
7) Eschenbach, D. A., Davick, P. R., Williams, B. L., Klebanoff, S. J., Young-Smith, K., Critchlow, C. M., & Holmes, K. K. (1989). Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. Journal of Clinical Microbiology, 27(2), 251–256 http://jcm.asm.org/content/27/2/251.full.pdf+html
8) Mirmonsef P. et al. (2015) Exploratory comparison of vaginal glycogen and Lactobacillus levels in premenopausal and postmenopausal women Menopause 22(7):702-9. doi:10.1097/GME.0000000000000397 http://www.ncbi.nlm.nih.gov/pubmed/25535963
9) Danielsson, Dan Teigen, Per Kristen Moi, Harald The genital econiche: focus on microbiota and bacterial vaginosis Annals of the New York Academy of Sciences 1230 (1) 1749-6632 http://dx.doi.org/10.1111/j.1749-6632.2011.06041.x
11) Evidence that vaginal lactobacilli do not ferment glycogen Stewart-Tull (1964) American Journal of Obstetrics & Gynecology , Volume 88 , Issue 5 , 676 - 679 http://www.researchgate.net/publication/9452627_Evidence_that_vaginal_lactobacilli_do_not_ferment_glycogen
12) Wylie JG, Henderson A (1969) Identity and glycogen-fermenting ability of lactobacilli isolated from the vagina of pregnant women. J Med Microbiol 2:363-6 http://gal.redplusalus.com/piwigo/galleries/file_uploader/pdf/medmicro_2_3_363.pdf
13) Martín R et al. (2998) Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates Int Microbiol. 11(4):261-6. http://www.im.microbios.org/1104/IM1104_0261.pdf
14) Spear, Gregory T. et al. (2014) Human α-amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus Journal of Infectious Diseases DO - 10.1093/infdis/jiu231 N1 http://jid.oxfordjournals.org/content/early/2014/05/14/infdis.jiu231.full
15) Mirmonsef, P., Hotton, A. L., Gilbert, D., Burgad, D., Landay, A., Weber, K. M., … Spear, G. T. (2014). Free Glycogen in Vaginal Fluids Is Associated with Lactobacillus Colonization and Low Vaginal pH. PLoS ONE, 9(7), e102467. http://doi.org/10.1371/journal.pone.0102467
16), 50) Holmes, King K. and Chen, Kirk C. S. and Lipinski, Carolyn M. and Eschenbach, David A. Vaginal Redox Potential in Bacterial Vaginosis (Nonspecific Vaginitis, (1985) vol 152(2), pages 379-382, doi = {10.1093/infdis/152.2.379 http://jid.oxfordjournals.org/content/152/2/379.abstract
17) Jarosik, G. P., Land, C. B., Duhon, P., Chandler, R., & Mercer, T. (1998). Acquisition of Iron by Gardnerella vaginalis. Infection and Immunity, 66(10), 5041–5047 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC108627/
18) Halliwell, Barry (2000) The antioxidant paradox The Lancet VL 355 (9210) 1179-1180 doi: 10.1016/S0140-6736(00)02075-4 http://oxfordindex.oup.com/view/10.1093/oi/authority.20110803095417247
19), 47) Srinivasan, Sujatha and Morgan, Martin T. and Fiedler, Tina L. and Djukovic, Danijel and Hoffman, Noah G. and Raftery, Daniel and Marrazzo, Jeanne M. and Fredricks, David N. (2015) Metabolic Signatures of Bacterial Vaginosis vol 6(2) doi: 10.1128/mBio.00204-15 http://mbio.asm.org/content/6/2/e00204-15.full
20) Nugent, R. P., Krohn, M. A., & Hillier, S. L. (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology, 29(2), 297–301 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC269757/
21) Spiegel, C. A., Amsel, R., & Holmes, K. K. (1983). Diagnosis of bacterial vaginosis by direct gram stain of vaginal fluid. Journal of Clinical Microbiology, 18(1), 170–177 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC270763/
22) Sha, B. E., Chen, H. Y., Wang, Q. J., Zariffard, M. R., Cohen, M. H., & Spear, G. T. (2005). Utility of Amsel Criteria, Nugent Score, and Quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for Diagnosis of Bacterial Vaginosis in Human Immunodeficiency Virus-Infected Women. Journal of Clinical Microbiology, 43(9), 4607–4612. http://doi.org/10.1128/JCM.43.9.4607-4612.2005
23) Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S. K., McCulle, S. L., … Forney, L. J. (2011). Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl 1), 4680–4687. http://doi.org/10.1073/pnas.1002611107
24) Kim, T. K., Thomas, S. M., Ho, M., Sharma, S., Reich, C. I., Frank, J. A., … Wilson, B. A. (2009). Heterogeneity of Vaginal Microbial Communities within Individuals . Journal of Clinical Microbiology, 47(4), 1181–1189. http://doi.org/10.1128/JCM.00854-08
25) Sautter, R. L., & Brown, W. J. (1980). Sequential vaginal cultures from normal young women. Journal of Clinical Microbiology, 11(5), 479–484 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC273436/
26), 43) Yeoman, C. J., Yildirim, S., Thomas, S. M., Durkin, A. S., Torralba, M., Sutton, G., … Wilson, B. A. (2010). Comparative Genomics of Gardnerella vaginalis Strains Reveals Substantial Differences in Metabolic and Virulence Potential. PLoS ONE, 5(8), e12411. http://doi.org/10.1371/journal.pone.0012411
27) Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C., Eschenbach, D., & Holmes, K. K. (1983). Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. The American Journal of Medicine, 74(1), 14–22. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6600371
28) Centers for Disease Control and Prevention et al., 2006 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5511a1.htm
29) Rifkin, S. B., Smith, M. R., Brotman, R. M., Gindi, R. M., & Erbelding, E. J. (2009). Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception, 80(1), 63–7. http://doi.org/10.1016/j.contraception.2009.01.008
30) Bradshaw, Catriona S. and Morton, Anna N. and Hocking, Jane and Garland, Suzanne M. and Morris, Margaret B. and Moss, Lorna M. and Horvath, Leonie B. and Kuzevska, Irene and Fairley, Christopher K. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence {2006} vol 193 {11}, 1478-1486 doi = {10.1086/503780 http://jid.oxfordjournals.org/content/193/11/1478.long
31) Suzanna C Francis, Clare Looker, Judith Vandepitte, Justine Bukenya, Yunia Mayanja, Susan Nakubulwa, Peter Hughes, Richard J Hayes, Helen A Weiss, Heiner Grosskurth (2015) Bacterial vaginosis among women at high risk for HIV in Uganda: high rate of recurrent diagnosis despite treatment Sex Transm Infect sextrans- doi:10.1136/sextrans-2015-052160 http://sti.bmj.com/content/early/2015/08/07/sextrans-2015-052160.full
32) Myer, Landon., Kuhn, Louise., Denny, Lynette., Wright, Jr, Thomas C. Recurrence of Symptomatic Bacterial Vaginosis 12 Months after Oral Metronidazole Therapy in HIV‐Positive and ‐Negative Women 2006/12/15 Journal of Infectious Diseases 1797-1799 194(12) 10.1086/509625 http://jid.oxfordjournals.org/content/194/12/1797.short
33) Ma, B., Forney, L. J., & Ravel, J. (2012). The vaginal microbiome: rethinking health and diseases. Annual Review of Microbiology, 66, 371–389. http://doi.org/10.1146/annurev-micro-092611-150157
34) Drell, T., Lillsaar, T., Tummeleht, L., Simm, J., Aaspõllu, A., Väin, E., … Metsis, M. (2013). Characterization of the Vaginal Micro- and Mycobiome in Asymptomatic Reproductive-Age Estonian Women. PLoS ONE, 8(1), e54379. http://doi.org/10.1371/journal.pone.0054379
35) Donders, G. (2010). Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: a review. Obstetrical & Gynecological Survey, 65(7), 462–73. http://www.ncbi.nlm.nih.gov/pubmed?Db=pubmed&Cmd=Retrieve&list_uids=20723268&dopt=abstractplus
36) Brocklehurst, P., Gordon, A., Heatley, E., & Milan, S. J. (2013). Antibiotics for treating bacterial vaginosis in pregnancy. The Cochrane Database of Systematic Reviews, 1, CD000262. http://doi.org/10.1002/14651858.CD000262.pub4
37) DAVID N. FREDRICKS AND DAVID A. RELMAN. (1996). Cli Micr Rev 1996 Sequence-Based Identification of Microbial Pathogens a Reconsideration of Koch Postulates.pdf. CLINICAL MICROBIOLOGY REVIEWS, 9, 18–33. http://cmr.asm.org/content/9/1/18.long
38) Braundmeier, A. G., Lenz, K. M., Inman, K. S., Chia, N., Jeraldo, P., Walther-António, M. R. S., … White, B. A. (2015). Individualized medicine and the microbiome in reproductive tract. Frontiers in Physiology, 6, 97. http://doi.org/10.3389/fphys.2015.00097
39) Kacerovsky, M., Vrbacky, F., Kutova, R., Pliskova, L., Andrys, C., Musilova, I., … Nekvindova, J. (2015). Cervical Microbiota in Women with Preterm Prelabor Rupture of Membranes. PLoS ONE, 10(5), e0126884. http://doi.org/10.1371/journal.pone.0126884
40), 48) Yeoman, C. J., Thomas, S. M., Miller, M. E. B., Ulanov, A. V., Torralba, M., Lucas, S., … White, B. A. (2013). A Multi-Omic Systems-Based Approach Reveals Metabolic Markers of Bacterial Vaginosis and Insight into the Disease. PLoS ONE, 8(2), e56111. http://doi.org/10.1371/journal.pone.0056111
41) Srinivasan, S., Morgan, M. T., Fiedler, T. L., Djukovic, D., Hoffman, N. G., Raftery, D., … Fredricks, D. N. (2015). Metabolic Signatures of Bacterial Vaginosis. mBio, 6(2), e00204–15. http://doi.org/10.1128/mBio.00204-15
42) Yanaka, N. (2007). Mammalian glycerophosphodiester phosphodiesterases. Bioscience, Biotechnology, and Biochemistry, 71(8), 1811–8. http://doi.org/10.1271/bbb.70062
44) Lewis, Warren G. and Robinson, Lloyd S. and Gilbert, Nicole M and Perry, Justin C. and Lewis, Amanda L. (2013) Degradation, Foraging, and Depletion of Mucus Sialoglycans by the Vagina-adapted Actinobacterium Gardnerella vaginalis vol 288(17) 12067-12079 doi = {10.1074/jbc.M113.453654 http://www.jbc.org/content/288/17/12067.long
45) Myziuk, Linda and Romanowski, Barbara and Johnson, Stephen C. (2003) BVBlue Test for Diagnosis of Bacterial Vaginosis}, vol 41(5) 1925-1928 doi = {10.1128/JCM.41.5.1925-1928.2003 http://jcm.asm.org/content/41/5/1925.full
46) Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S. K., McCulle, S. L., … Forney, L. J. (2011). Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl 1), 4680–4687. http://doi.org/10.1073/pnas.1002611107
51) Gillet, E., Meys, J. F. A., Verstraelen, H., Verhelst, R., De Sutter, P., Temmerman, M., & Broeck, D. V. (2012). Association between Bacterial Vaginosis and Cervical Intraepithelial Neoplasia: Systematic Review and Meta-Analysis. PLoS ONE, 7(10), e45201. http://doi.org/10.1371/journal.pone.0045201






